Mimetikos Preludium™ Software
Cutting-Edge Software for Aerosol Deposition and Pharmacokinetic Simulations.

Mimetikos Preludium™ Software – Overview
Mimetikos Preludium™ is an advanced software solution meticulously designed for researchers, pharmaceutical developers, and toxicologists who need to model and simulate the behavior of aerosols within the human respiratory system. The software is a comprehensive tool that not only simulates how particles deposit in various regions of the lungs but also integrates pharmacokinetic (PK) simulations to predict how these particles are absorbed, distributed, metabolized, and excreted by the body. This dual functionality makes Preludium a vital resource for optimizing the development and evaluation of inhalation therapies, as well as for conducting sophisticated inhalation toxicology assessments.
Detailed Features and Key Advantages
1. Deposition Modeling:
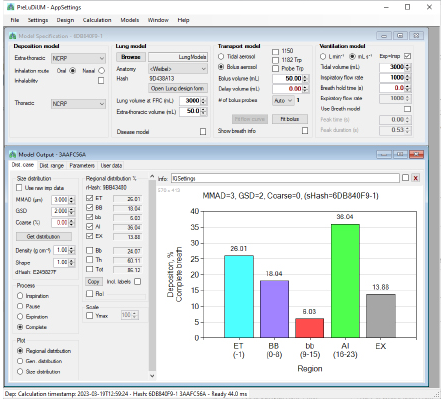
- Advanced Simulation Algorithms: Mimetikos Preludium™ employs state-of-the-art engineering equations to simulate aerosol behavior under various conditions. It meticulously accounts for processes such as particle impaction, sedimentation, and diffusion, which are critical for understanding aerosol deposition in different lung regions, including the extrathoracic, thoracic, and alveolar areas.
- Customizable Lung Models: The software provides users with the ability to select and customize lung models based on specific research needs. This includes altering parameters like lung volume, airway geometry, and breathing patterns to reflect realistic scenarios or hypothetical conditions.
- Multi-Stage Deposition: Preludium allows users to model deposition across multiple stages of the respiratory tract, from the mouth and throat to the deepest regions of the lungs. This detailed approach ensures a precise understanding of how various factors influence where and how particles deposit.
2. Integrated Pharmacokinetic Module:
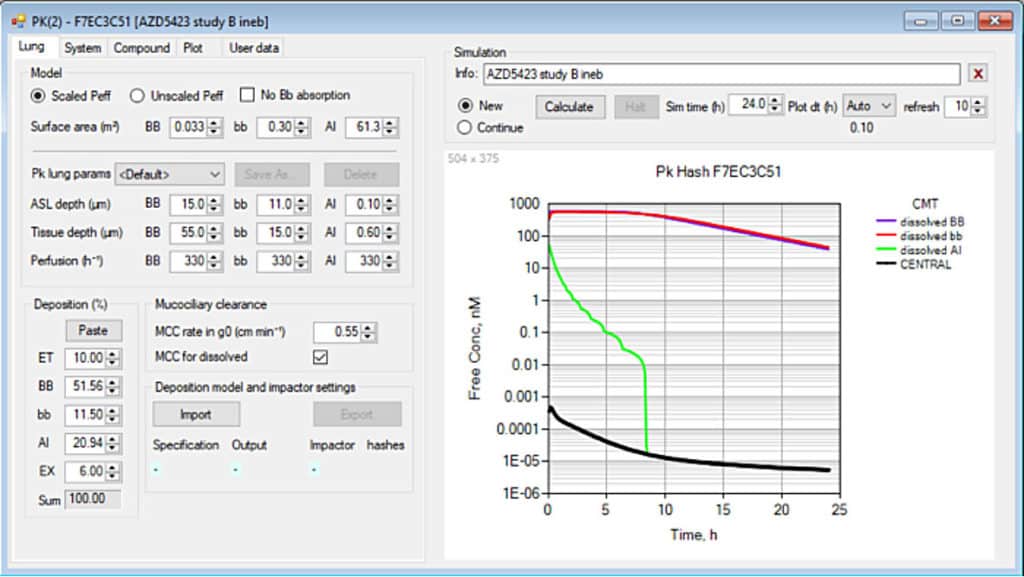
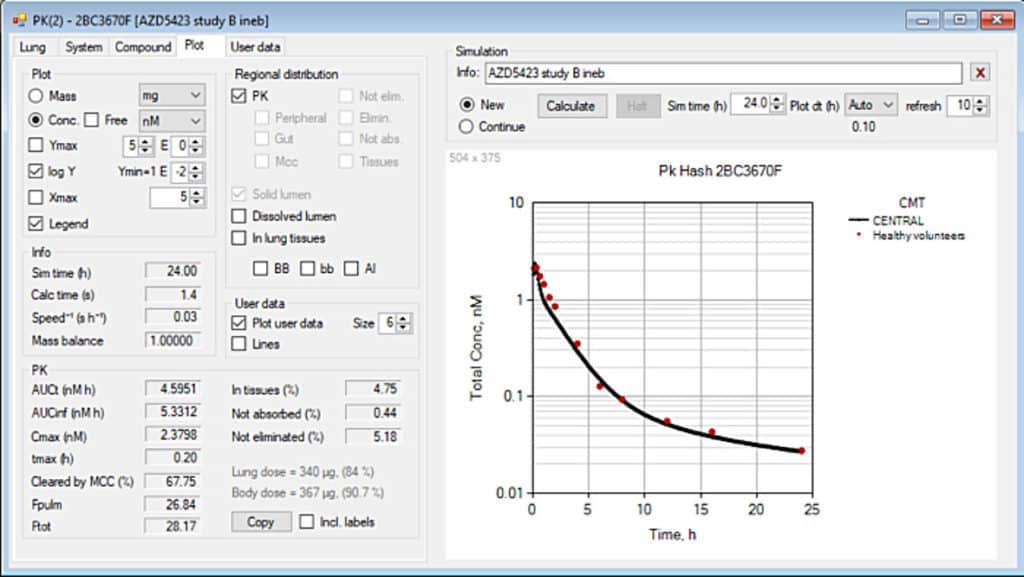
- In-Depth PK Simulations: The integrated pharmacokinetic module offers users the ability to simulate the entire lifecycle of inhaled drugs post-deposition. This includes predicting the absorption of the drug into the bloodstream, its distribution throughout the body, its metabolic breakdown, and eventual excretion.
- Versatile Drug Delivery Analysis: The PK module is highly adaptable, supporting various forms of drug delivery, including oral, nasal, and pulmonary routes. This versatility is crucial for researchers aiming to optimize delivery methods and improve therapeutic outcomes.
- Chainable Simulations: Users can chain together simulations for deposition and pharmacokinetics, enabling a seamless workflow from particle deposition to drug effect prediction. This feature is particularly useful in optimizing dosing regimens and understanding the impact of different delivery techniques.
3. Automation of Complex Calculations:
- Streamlined Workflow: Preludium’s automation capabilities significantly reduce the time and effort required to perform complex simulations. Users can automate repetitive tasks and batch process large datasets, making it easier to handle extensive research projects with multiple variables.
- Error Reduction and Consistency: By automating calculations, the software minimizes the risk of human error, ensuring that results are consistent and reliable across different simulation runs. This consistency is critical in research settings where reproducibility is paramount.
- Interactive Output Management: The software provides an interactive environment for managing output data. Users can customize how results are displayed, enabling quick identification of key outcomes and facilitating easier interpretation of complex data.
4. User-Friendly Interface with Advanced Control:
- Intuitive Design: Mimetikos Preludium™ features a well-organized interface that guides users through the setup, execution, and analysis of simulations. The layout is designed to accommodate both beginners and experts, with easy access to advanced features for those who need them.
- Comprehensive Documentation and Support: The software comes with detailed user manuals, tutorials, and support resources, ensuring that users can quickly get up to speed and make the most of the software’s capabilities. This support is crucial for maintaining productivity and ensuring that users can overcome any challenges they encounter.
- Multi-Tab Layout for Efficiency: The interface includes multiple tabs for different aspects of the simulation process, such as specification, input, output, and reporting. This layout allows users to efficiently navigate between different stages of their projects, enhancing workflow efficiency.
5. Extensive Customization and Flexibility:
- Tailored Simulations: Users can customize nearly every aspect of their simulations, from the physical properties of aerosols (such as size, shape, and density) to the physiological parameters of the lung model. This flexibility is essential for conducting targeted research that meets specific project goals.
- Cross-Scenario Comparisons: Preludium supports the comparison of different scenarios side by side, allowing researchers to evaluate the impact of various changes in parameters on aerosol deposition and drug pharmacokinetics. This capability is invaluable for decision-making in drug development and toxicology assessments.
- Integration with External Tools: The software is compatible with widely used tools like Microsoft Excel, facilitating easy data import and export. This integration ensures that Preludium can be seamlessly incorporated into existing research workflows.
6. Robust Security and Reporting:
- Secure Data Handling: Preludium offers features for securing sensitive data, including the option to password-protect reports. This ensures that confidential research data remains protected throughout the project lifecycle.
- Comprehensive Reporting Tools: The software includes advanced reporting features, enabling users to generate detailed reports of their simulations. Reports can include both visual summaries (such as graphs and charts) and detailed numerical data, which can be exported in various formats for further analysis or presentation.
- Verification and Integrity Checks: For added security, the software includes options for verifying the integrity of exported data files. This ensures that the data has not been tampered with and remains consistent with the original simulation results.
Mimetikos Preludium™ – Conclusion
Mimetikos Preludium™ is a groundbreaking tool for anyone engaged in the study of aerosol deposition, drug delivery, and pharmacokinetics within the respiratory system. Its powerful simulation capabilities, combined with an intuitive interface and extensive customization options, make it an essential asset for research and development in respiratory science, pharmaceutical development, and toxicology. Whether you are optimizing a new inhalation therapy or studying the safety of airborne substances, Preludium offers the precision, flexibility, and reliability you need to achieve your research goals.
For further details or to arrange a demonstration:
Technical Specifications – Mimetikos Preludium™ Software
Mimetikos Preludium™ software is a Windows application written in MS Visual Studio (.NET) and runs on Windows XP or later. It is available for both 32 and 64-bit OS.
It does not require installation, only a licence that is both computer and user specific. The licence is sold by Emmace.
Dr. Olsson has more than 30 years of experience of inhaled formulations having spent about equal time on CMC and clinical pharmacology at his former employer, AstraZeneca. In 2015 he took a position as senior inhalation consultant at Emmace Consulting AB. His specialty is IVIVC with particular emphasis on predicting in vivo outcomes, such as lung disposition, from in vitro data, and is a well-known author and speaker on this topic. For many years he was part of the aerosol committees of USP and EP and member of industry groups such as IPAC-RS and EPAG. He was part of the teams developing the multi-stage liquid impinger, the next generation impactor and the OPC mouth-throat models. He is author and owner of the Mimetikos Preludium software.


